Welcome to Union-agrochemCrop Protection Technology (Shanghai) Co.,ltd
Toggle Navigation
|
FRAC 11, C3 Qol; strobilurin type: methoxyacrylate |
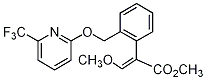 |
Common name picoxystrobine ((m) F-ISO); picoxystrobin (BSI, E-ISO) IUPAC name methyl (E)-3-methoxy-2-[2-(6-trifluoromethyl-2-pyridyloxymethyl)phenyl]acrylate
PHYSICAL CHEMISTRY
Composition Tech. is ≥95% (EU Rev. Rep.). Mol. wt. 367.3 M.f. C18H16F3NO4 Form Colourless powder; (tech. is a solid, with creamy colour). M.p. 75 °C V.p. 5.5 × 10-3 mPa (20 °C) Kow logP = 3.6 (20 °C) Henry 6.5 × 10-4 Pa m3 mol-1 (calc.) S.g./density 1.4 (20 °C) Solubility In water 3.1 mg/l (20 °C). In methanol 96, 1,2-dichloroethane, acetone, xylene and ethyl acetate >250 (all in g/l, 20 °C). Stability Stable at pH 5 and pH 7; DT50 c. 15 d (pH 9, 50 °C) (EU Rev. Rep.).
APPLICATIONS
Biochemistry Quinone outside Inhibitor. Inhibits mitochondrial respiration by blocking electron transfer at the Qo centre of cytochrome bc1. Mode of action Preventive and curative fungicide with unique distribution properties, including systemic (acropetal) and translaminar movement, diffusion in leaf waxes and molecular redistribution in air. Uses For broad-spectrum disease control, including Mycosphaerella graminicola, Phaeosphaeria nodorum, Puccinia recondita (brown rust), Helminthosporium tritici-repentis (tan spot) and Blumeria graminis f.sp. tritici (strobilurin-sensitive powdery mildew) in wheat; Helminthosporium teres (net blotch), Rhynchosporium secalis, Puccinia hordei (brown rust) and Erysiphe graminis f.sp. hordei (strobilurin-sensitive powdery mildew) in barley; Puccinia coronata and Helminthosporium avenae in oats; and Puccinia recondita and Rhynchosporium secalis in rye. Application rate typically 250 g/ha. Formulation types SC.
ENVIRONMENTAL FATE
Animals In rats, well absorbed, extensively metabolised and rapidly eliminated. Metabolism proceeds mainly by ester hydrolysis and glucuronide conjugation. Does not accumulate in meat or milk. Plants Residues in cereals are low (<0.01–0.20 mg/kg). Soil/Environment Rapidly degraded in soils, with CO2 as the major product; lab. DT50 (aerobic) 19–33 d; field dissipation DT50 3–35 d. Not mobile in soil under field conditions; Koc 790–1200 ml/g. Rapid dissipation in water indicates no chronic issues for aquatic organisms; water phase DT50 7–15 d (lab. and outdoor water sediment systems).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 for rats >2.12 mg/l at MMAD >4 μl. NOEL NOAEL (1 y and 90 d) for dogs 4.3 mg/kg b.w. daily. ADI (EC) 0.04 mg/kg b.w. [2003]. Other Non-genotoxic; no developmental toxicity potential (rats and rabbits); no reprotoxicity potential (rats); no carcinogenic potential (rats and mice).
ECOTOXICOLOGY
Birds LD50 for bobwhite quail >2250 mg/kg. Dietary LD50 (8 d) for bobwhite quail >5200 mg/kg. NOEC (21 w) for mallard ducks 1350 mg/kg. Fish LC50 (96 h) (two species) 65–75 μg/l. Daphnia EC50 (48 h) 18 μg/l. Algae EbC50 (72 h) for Selenastrum capricornutum 56 μg/l. Other aquatic spp. EC50 for Chironomus riparius 19 mg/kg (28 d, dosed to sediment), 140 μg/l (25 d, dosed to water). Bees LD50 (48 h, contact and oral) >200 μg/bee. Worms LC50 (14 d) for Eisenia foetida 6.7 mg/kg soil. Other beneficial spp. Lab. and field tests with 6 species of non-target arthropods indicate low risk to populations. LR50 (7 d) for Typhlodromus pyri 12.6 g/ha; (2 d) for Aphidius rhopalosiphi 280 g/ha.