Welcome to Union-agrochemCrop Protection Technology (Shanghai) Co.,ltd
Toggle Navigation
|
FRAC 11, C3 Qol; strobilurin type: methoxyacrylat |
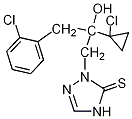 |
Common name prothioconazole (BSI, E-ISO, (m) F-ISO) IUPAC name 2-[(2RS)-2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]-2H-1,2,4-triazole-3(4H)-thione
PHYSICAL CHEMISTRY
Composition Tech. is ≥97%. Mol. wt. 344.3 M.f. C14H15Cl2N3OS Form White to light beige crystalline powder. M.p. 139.1–144.5 °C B.p. 487±50 °C (calc.) V.p. <<4 × 10-4 mPa (20 °C) Kow logP = 4.05 (unbuffered, 20 °C), 4.16 (pH 4), 3.82 (pH 7), 2.00 (pH 9) Henry <<3 × 10-5 Pa m3 mol-1 S.g./density 1.36 (20 °C) Solubility In water 0.005 (pH 4), 0.3 (pH 8), 2.0 (pH 9), (all in g/l, 20 °C). In n-heptane <0.1, xylene 8, n-octanol 58, isopropanol 87, acetonitrile 69, DMSO 126, dichloromethane 88, ethyl acetate, polyethylene glycol and acetone all >250 (all in g/l, 20 °C). Stability Stable at ambient temperature. Stable to hydrolysis at pH 4–9. Rapidly photodegraded to prothioconazole-desthio in water. pKa 6.9
APPLICATIONS
Biochemistry Sterol demethylation (ergosterol biosynthesis) inhibitor. Mode of action Systemic fungicide with protective, curative, eradicative and long-lasting activity. Uses For control of diseases such as eyespot (Pseudocercosporella herpotrichoides), Fusarium ear blight (Fusarium spp., Microdochium nivale), leaf blotch diseases (Septoria tritici, Leptosphaeria nodorum, Pyrenophora spp., Rhynchosporium secalis, etc.), rust (Puccinia spp.) and powdery mildew (Blumeria graminis), by foliar application, in wheat, barley and other crops. As a seed dressing, for control of Ustilago spp., Tilletia spp., Fusarium spp. and Microdochium nivale. Formulation types EC; FS; SC.
ANALYSIS
Product by hplc/uv. Residues by hplc ms/ms. Methods for determination are available from Bayer CropScience.
ENVIRONMENTAL FATE
Animals Prothioconazole is rapidly and extensively absorbed and rapidly eliminated, predominantly via faeces. It does not show potential for accumulation. Prothioconazole is extensively metabolised, with the major metabolic reactions being conjugation with glucuronic acid, oxidative hydroxylation of the phenyl moiety and desulfuration. Plants The metabolism of prothioconazole proceeds through oxidative and cleavage reactions. The major metabolites are prothioconazole-desthio and triazolylalanine, triazolylhydroxypropionic acid and triazolylacetic acid. No free 1,2,4-triazole was detected in any plant matrix. Soil/Environment Prothioconazole is rapidly degraded to prothioconazole-desthio and prothioconazole-S-methyl. Parent compound and metabolites show low potential for leaching or accumulation. For prothioconazole, prothioconazole-desthio and prothioconazole-S-methyl, soil DT50 (lab., 20 °C) 0.07–1.3 d, 7–34 d, and 6–46 d, respy.; Koc 1765 ml/g, 523–625 ml/g and 1974–2995 ml/g, respy. Prothioconazole degraded rapidly in water/sediment systems under aerobic conditions (DT50 for total system 2–3 d); major metabolites are prothioconazole-desthio and 1,2,4-triazole (detected in the water layer) and prothioconazole-S-methyl (in sediment).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >6200 mg/kg. Skin and eye Acute dermal LD50 for rats >2000 mg/kg. Not an eye or skin irritant. Not a skin sensitiser. Inhalation LC50 for rats >4990 mg/m3 air. NOEL Short term oral NOAEL (13 w) for dogs 25 mg/kg b.w. daily; chronic NOAEL (2 y) for rats 5 mg/kg b.w. daily (EFSA Sci. Rep.). For prothioconazole-desthio [2-(1-chlorocyclopropyl)1-(2-chlorophenyl)-3-(1,2,4-triazol-1-yl)-propan-2-ol], short term NOAEL for dogs 1.6 mg/kg daily; chronic NOAEL for rats 1.1 mg/kg daily. ADI (EC) for prothioconazole 0.05 mg/kg b.w., for the desthio metabolite 0.01 mg/kg b.w. [2007]; (EPA) for prothioconazole no RfD established, for the desthio metabolite aRfD 0.002, cRfD 0.001 mg/kg b.w. [2007]. Other No genotoxic effects; no embryotoxic or teratogenic potential. EC classification Proposed classification Xn N; R51, R53.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 (5 d) for bobwhite quail >5000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 1.83 mg/l. Daphnia Acute LC50 (48 h) 1.30 mg/l. Algae For Pseudokirchneriella subcapitata, subchronic EbC50 1.10 mg/l, ErC50 2.18 mg/l. Bees Not harmful; LD50 (oral) >71 μg/bee; (contact) >200 μg/bee. Worms LC50 (14 d) for earthworms >1000 mg/kg dry soil. Other beneficial spp. No effects on non-target arthropods or soil organisms.