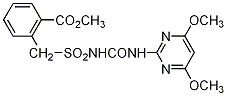 NOMENCLATURE
Common name
NOMENCLATURE
Common name bensulfuron-methyl
IUPAC name methyl α-(4,6-dimethoxypyrimidin-2-ylcarbamoylsulfamoyl)-
o-toluate
Chemical Abstracts name methyl 2-[[[[[(4,6-dimethoxy-2-pyrimidinyl)-amino]carbonyl]amino]sulfonyl]methyl]benzoate
CAS RN [83055–99–6] EC no. 401–340–6
PHYSICAL CHEMISTRY
Composition Tech. is 97.5%.
Mol. wt. 410.4
M.f. C
16H
18N
4O
7S
Form White odourless solid.
M.p. 185–188 °C; (
tech., 179.4 °C)
V.p. 2.8 × 10
-9 mPa (25 °C)
Kow logP = 2.18 (
pH 5), 0.79 (pH 7), –0.99 (pH 9) (all 25 °C) (
EPA Fact Sheet)
Henry 2 × 10
-11 Pa m
3 mol
-1 (calc.)
S.g./density 1.49 (20 °C)
Solubility In water 2.1 (
pH 5), 67 (pH 7), 3100 (pH 9) (all in mg/l, 25 °C). In acetone 5.10, acetonitrile 3.75, dichloromethane 18.4, ethyl acetate 1.75,
n-heptane 3.62 × 10
-4, xylene 0.229 (all in g/l, 20 °C).
Stability Aqueous solutions are most stable under slightly alkaline conditions (
pH 8), and slowly degrade under acidic conditions;
DT50 6 d (pH 4), stable (pH 7), 141 d (pH 9) (all 25 °C).
pKa 5.2
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (
ALS or
AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity is due to rapid metabolism in the crop. Metabolic basis of selectivity reviewed (M. K. Koeppe & H. M. Brown,
Agro-Food-Industry, 1995,
6, 9–14).
Mode of action Selective systemic herbicide, absorbed by the foliage and roots, with rapid translocation to the meristematic tissues.
Uses Selective pre- and post-emergence control of annual and perennial weeds and sedges (e.g.
Butomus umbellatus,
Scirpus maritimus,
Scirpus mucronatus,
Alisma plantago-aquatica,
Sparganium erectum,
Cyperus spp.,
Typha spp., etc.) in flooded or wetland rice, at 48–60 g/ha.
Formulation types WG;
WP.
ENVIRONMENTAL FATE
Animals Almost completely biotransformed and rapidly excreted in urine and faeces of rats and goats. The primary metabolic pathways include hydroxylation and
O-demethylation (
FAO Specification).
Plants After uptake by rice, converted to a non-herbicidal metabolite.
Soil/Environment DT50 (
ave.) 88.5 d on Flanagan and Keyport silt loam soils. DT
50 in water 16–21 d for Italian soils.
MAMMALIAN TOXICOLOGY
Oral Acute oral
LD50 for rats >5000 mg/kg.
Skin and eye Acute percutaneous
LD50 for rabbits >2000 mg/kg. Non-irritant to skin and eyes. Not a skin sensitiser (Buehler method).
Inhalation LC50 (4 h) for rats 5 mg/l air.
NOEL (1 y) for male dogs 21.4 mg/kg b.w. daily; reproduction (2 generation)
NOEL for male rats 20 mg/kg b.w. daily; teratogenicity NOEL for rabbits 300 mg/kg b.w.. daily. No developmental toxicity or teratogenicity.
ADI (
EC) 0.2 mg/kg
b.w. [2008]; (
EPA)
cRfD 0.20 mg/kg b.w. [1991]; AOEL (1 y, dog) 0.12 mg/kg/d.
Toxicity class WHO (a.i.) U
EPA (formulation) IV EC classification R43| N;
R51,
R53
ECOTOXICOLOGY
Birds Acute oral
LD50 for mallard ducks >2510 mg/kg. Dietary
LC50 (8 d) for bobwhite quail, mallard ducks >5620 mg/l.
Fish LC50 (96 h) for rainbow trout >66, bluegill sunfish >120 mg/l.
Daphnia LC50 (48 h) >130 mg/l.
Algae EC50 (72 h) for
Selenastrum capricornutum 0.020 mg/l.
Other aquatic spp. EC50 (14 d) for
Lemna gibba 0.0008 mg/l.
Bees LD50 (oral) >51.41 μg/bee; (contact) >100 μg/bee.
Worms LC50 >1000 mg/kg soil.
Other beneficial spp. NOEC for
Aphidius rhopalosiphi and
Typhlodromus pyri ≥1000 g formulated product/
ha in a volume of 200 l/ha.