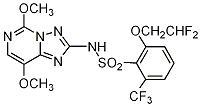
NOMENCLATURE
Common name pénoxsulame ((m) F-ISO); penoxsulam (BSI, E-ISO)
IUPAC name 3-(2,2-difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-α,α,α-trifluorotoluene-2-sulfonamide; 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide
Chemical Abstracts name 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide
CAS RN [219714–96–2]
PHYSICAL CHEMISTRY
Composition Purity 98% nominal. Mol. wt. 483.4 M.f. C16H14F5N5O5S Form Off-white solid, with a musty odour. M.p. 212 °C V.p. 9.55 × 10-11 mPa (25 °C) Kow logP = −0.354 (unbuffered water, 19 °C) S.g./density 1.61 (20 °C) Solubility In water 0.0049 (distilled), 0.00566 (pH 5), 0.408 (pH 7), 1.46 (pH 9) (all in g/l, 19 °C). In acetone 20.3, methanol 1.48, octanol 0.035, DMSO 78.4, NMP 40.3, 1,2-dichlorethane 1.99, acetonitrile 15.3 (all in g/l, 19 °C). Stability Stable to hydrolysis. Photolysis DT50 2 d. Storage stability >2 y. pKa 5.1 Other properties Not flammable or explosive.
APPLICATIONS
Biochemistry Branched chain amino acid (leucine, isoleucine and valine) synthesis (ALS or AHAS) inhibitor. Selectivity is based on differential metabolism to inactive metabolites. Mode of action Absorbed mainly via leaves, and secondarily via roots, and is translocated in both phloem and xylem. Symptoms include almost immediate growth inhibition, a chlorotic growing point with necrosis of the terminal bud, resulting in plant death in 2 to 4 weeks. Applied pre-emergence, post-emergence and water-applied. Uses Provides control of Echinochloa spp., as well as many broad-leaved, sedge and aquatic weeds (such as Alisma plantago-aquatica, Ammania coccinea, Cyperus difformis and Scirpus mucronatus) in rice. Penoxsulam provides residual weed control, depending on soil type and use rate, and is rainfast within 1 hour of application. In tropical rice, application will be at 10–15 g/ha; in temperate rice, 20–50 g/ha. Primary use will be a post-emergence application in dry-seeded, water-seeded and transplanted rice. Formulation types GR; OD; SC.
ENVIRONMENTAL FATE
Animals Rapidly excreted, with low potential to accumulate. Plants Following post-emergence foliar application to glasshouse plants, DT50 in indica rice 0.6 d, japonica rice 1.4 d, Echinochloa 4.4 d. Penoxsulam is first metabolised to the 5-hydroxy derivative, which is inactive. No penoxsulam residues are found in harvested rice grain (limit of determination 0.002 mg/kg). Soil/Environment In water, degradation is mainly by photolysis and by biological means. Aqueous photolysis DT50 2 d; soil photolysis DT50 19 d. Under global water-seeded rice field conditions, DT50 (ave.) 6.5 d (4–10 d); under dry-seeded rice conditions, DT50 (ave.) 14.6 d (13–16 d). In EU, under water-seeded field conditions, DT50 (ave.) 5.9 d (5.6–6.1 d). In soil, degradation is mainly microbiological; lab. DT50 (aerobic, 20 °C) 32 d (22–58 d), (anaerobic, 20 °C) 6.6 d. Likely to be very mobile, but not very persistent, in either aqueous or terrestrial environments; produces 11 major degradation products, some of which are more persistent than penoxsulam (EPA Fact Sheet).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Mild, transient eye irritation; very slight, transient skin irritation (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 for rats >3.50 mg/l (highest attainable concentration). NOEL for rats 500 mg/kg b.w. daily (maternal), 1000 mg/kg b.w. daily (embryo-foetal). ADI (EPA) cRfD 0.147 mg/kg b.w. [2004]. Other Not mutagenic in Ames, CHO-HGPRT, micronucleus and mouse lymphoma tests. Toxicity class WHO (a.i.) U EPA (formulation) III (GR, SC)
ECOTOXICOLOGY
Penoxsulam has low toxicity to fish, birds, terrestrial and aquatic invertebrates, with low to moderate toxicity to aquatic plants. Birds LD50 for mallard ducks >2000, bobwhite quail >2025 mg/kg b.w. Dietary LC50 (8 d) for mallard ducks >4310, bobwhite quail >4411 ppm. Fish LC50 (96 h) for common carp >101, bluegill sunfish >103, rainbow trout >102, silverside >129 mg/l. NOEC (36 d) for fathead minnows 10.2 mg/l. Daphnia EC50 (24 h and 48 h) >98.3 mg/l. Algae EC50 (120 h) for freshwater diatoms >49.6, blue-green algae 0.49 mg/l; (96 h) for freshwater green algae 0.086 mg/l. Other aquatic spp. EC50 (14 d) for Lemna gibba 0.003 mg/l. Bees LD50 (48 h, oral) for honeybees >110 μg/bee; (48 h, contact) >100 μg/bee. Worms LC50 (7 d and 14 d) >1000 mg/kg. Other beneficial spp. LR50 (glass plate test) for predatory mites 7.46, parasitic wasps and green lacewing >40 g/ha. Extended laboratory test at 40 g/ha: predatory mite mortality 0%, effect on fecundity 8.2%; parasitic wasp mortality 0%, effect on fecundity 26%. Soil microbial NOEC >500 g/ha.